Q#19.14
Boiling Water at High Pressure. When water is boiled at a pressure of 2.00 atm, the heat of vaporization is 2.20 $\times 10^6$ J/kg and the boiling point is 120$^0$C. At this pressure, 1.00 kg of water has a volume of 1.00 $\times 10^{-3 \ m^3}$ and 1.00 kg of steam has a volume of 0.824 $m^3$.
(a) Compute the work done when 1.00 kg of steam is formed at this temperature.
(b) Compute the increase in internal energy of the water.
Answer:
We can use ΔU = Q − W.
For a constant pressure process, W = pΔV
Given: Q = 2.20 $\times 10^6$ J/kg, ; Q > 0 since this amount of heat goes into the water.
p = 2.00 atm = 2.03 × $10^5$ Pa
$V_1$=1.00 $\times 10^{-3} m^3$ and $V_2$ = 0.824 $m^3$
(a) W = pΔV = (2.03 × $10^5$ Pa)(0.824 $m^3-$ 1.00 $\times 10^{-3} m^3$)
W = 1.67 $\times 10^5$ J
(b) ΔU = Q − W
ΔU = 2.20 $\times 10^6 \ J -$ 1.67 $\times 10^5$ J
ΔU = 2.03 $\times 10^5$ J
2.20 $\times 10^6$ J of energy enters the water. 1.67 $\times 10^5$ J of energy leaves the materials through expansion work and the remainder stays in the material as an increase in internal energy.
Q#19.15
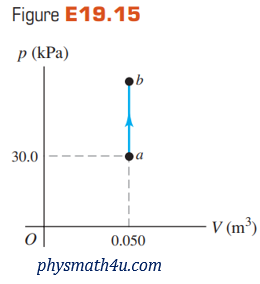
An ideal gas is taken from a to b on the pV-diagram shown in Fig. E19.15. During this process, 700 J of heat is added and the pressure doubles.
(a) How much work is done by or on the gas? Explain.
(b) How does the temperature of the gas at a compare to its temperature at b? Be specific.
(c) How does the internal energy of the gas at a compare to the internal energy at b? Again, be specific and explain.
Answer:Q#19.16
A system is taken from state a to state b along the three paths shown in Fig. E19.16.
(a) Along which path is the work done by the system the greatest? The least?
(b) If $U_b$ > $U_a$ along which path isthe absolute value |Q| of the heat transfer the greatest? For this path, is heat absorbed or liberated by the system?
Answer:
Apply ΔU = Q − W. $W$ is the area under the path in the pV-plane.
W > 0 when V increases.
(a) The greatest work is done along the path that bounds the largest area above the V-axis in the p-V plane, which is path 1. The least work is done along path 3.
(b) 0 W > in all three cases; ΔU = Q − W, so Q > 0 for all three, with the greatest Q for the greatest work, that along path 1. When 0, Q > 0 heat is absorbed.
ΔU is path independent and depends only on the initial and final states. W and Q are path dependent and can have different values for different paths between the same initial and final states.
Q#19.17
A thermodynamic system undergoes a cyclic process as shown in Fig. E19.17. The cycle consists of two closed loops: I and II.
(a) Over one complete cycle, does the system do positive or negative work?
(b) In each of loops I and II, is the net work done by the system positive or negative?
(c) Over one complete cycle, does heat flow into or out of the system? (d) In each of loops I and II, does heat flow into or out of the system?
Answer:
ΔU = Q − W. W is the area under the path in the pV-diagram. When the volume increases, W > 0
For a complete cycle, ΔU = 0.
(a) and (b) The clockwise loop (I) encloses a larger area in the p-V plane than the counterclockwise loop (II). Clockwise loops represent positive work and counterclockwise loops negative work, so $W_I$ > 0 and $W_{II}$ < 0 Over one complete cycle, the net work $W_1+W{II}$ > 0, and the net work done by the system is positive.
(c) For the complete cycle, ΔU = Q − W and so From part (a), 0, W > so Q > 0, and heat flows into the system.
(d) Consider each loop as beginning and ending at the intersection point of the loops. Around each loop, ΔU = 0, so Q = W; then, $Q_I = W_I$ > 0 and $Q_{II}=W_{II}$ < 0. Heat flows into the system for loop I and out of the system for loop II.
W and Q are path dependent and are in general not zero for a cycle.


Post a Comment for " Internal Energy and the First Law of Thermodynamics Problems and Solutions 3"