Q#19.8
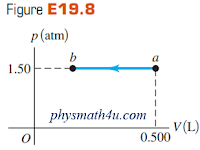
Figure E19.8 shows a pV-diagram for an ideal gas in which its absolute temperature at b is one-fourth of its absolute temperature at a.
(a) What volume does this gas occupy at point b?
(b) How many joules of work was done by or on the gas in this process? Was it done by or on the gas?
(c) Did the internal energy of the gas increase or decrease from a to b? How do you know?
(d) Did heat enter or leave the gas from a to b? How do you know?
Answer:
The gas is undergoing an isobaric compression, so its temperature and internal energy must be decreasing.
The pV diagram shows that in the process the volume decreases while the pressure is constant.
1 L = $10^{-3} \ m^3$ and 1 atm = 1.013 $\times 10^5$ Pa.
(a) pV = nRT with n, R and p are constant so,
$\frac{V}{T}=\frac{nR}{p}$ and
$\frac{V_a}{T_a}=\frac{V_b}{T_b}$
$V_b=V_a\frac{T_b}{T_a}$
$V_b=(0.500 \ L)\left(\frac{T_a/4}{T_a}\right)=0.125 \ L$
(b) For a constant pressure process,
W = pΔV = (1.50 atm)(0.125 L − 0.500 L) = − 0.5625 atm.L and
W = (−0.5625 atm.L )(1.013 $\times 10^5$ Pa/atm)($10^{-3}$ $m^3/L$)
W = −57.0 J
W is negative since the volume decreases. Since W is negative, work is done on the gas.
(c) For an ideal gas, U = nCT so U decreases when T decreases. The internal energy of the gas decreases because the temperature decreases.
(d) For a constant pressure process, Q = n $C_p\Delta T$
T decreases so ΔT is negative and Q is therefore negative. Negative Q means heat leaves the gas.
W = nRΔT and Q = n $C_p\Delta T$. $C_p$ > R so more energy leaves as heat than is added by work done on the gas, and the internal energy of the gas decreases.
Q#19.9
A gas in a cylinder expands from a volume of 0.110 $m^3$ to $0.320 m^3$. Heat flows into the gas just rapidly enough to keep the pressure constant at 1.65 $\times 10^5$ Pa during the expansion. The total heat added is 1.15 $\times 10^5$ J.
(a) Find the work done by the gas.
(b) Find the change in internal energy of the gas.
(c) Does it matter whether the gas is ideal? Why or why not?
Answer:
We use, ΔU = Q − W and for a constant pressure process, W = pΔV
Given: Q = + 1.15 $\times 10^5$ J since heat enters the gas.
p = 1.65 $\times 10^5$ Pa
$V_1$ = 0.110 $m^3$ and $V_2$ = $0.320 m^3$
(a) the work done by the gas is
W = pΔV = (1.65 \times 10^5 \ Pa)(0.320 $m^3$ - 0.110 $m^3$)
W = 3.47 $\times 10^4$ J
(b) ΔU = Q − W = 1.15 $\times 10^5$ J − 3.47 $\times 10^4$ J
ΔU = 8.04 $\times 10^4$ J
(c) W = pΔV for a constant pressure process and ΔU = Q − W both apply to any material. The ideal gas law wasn’t used and it doesn’t matter if the gas is ideal or not.
Q#19.10
Five moles of an ideal monatomic gas with an initial temperature of 127 $^0$C expand and, in the process, absorb 1200 J of heat and do 2100 J of work. What is the final temperature of the gas?
Answer:
The type of process is not specified. We can use ΔU = Q − W because this applies to all processes. Calculate ΔU and then from it calculate ΔT.
Given: Q is positive since heat goes into the gas; Q = +1200 J.
W positive since gas expands; 2100 J.
ΔU = 1200 J − 2100 J = −900 J
We can also use
ΔU = $n\left(\frac{3}{2}R\right)$ΔT since this is true for any process for an ideal gas.
ΔT = $\frac{2\Delta U}{3nR}$
ΔT = $\frac{2(-900 \ J)}{3(5.00 \ mol)(8.3145 \ J/mol.K)}$
ΔT = −14.4 $^0C$
So, $T_2=\Delta T + T_2$
Post a Comment for " Internal Energy and the First Law of Thermodynamics Problems and Solutions "