Q#19.26
Propane gas ($C_3H_8$) behaves like an ideal gas with $\gamma$ = 1.127. Determine the molar heat capacity at constant volume and the molar heat capacity at constant pressure.
Answer:
We use $C_p=C_v+R$ and $\gamma = \frac{C_p}{C_v}$
$\gamma = \frac{C_v+R}{C_v}$
$\gamma = 1 + \frac{R}{C_v}$
$\gamma - 1 = \frac{R}{C_v}$
$C_v=\frac{R}{\gamma -1}=\frac{8.3145 \ J/mol.K}{1.127 - 1}=65.5 \ J/mol.K$
Then, $C_p$ = 65.6 J/mol.K + 8.3145 J/mol.K = 73.8 J/mol.K
Q#19.27
The temperature of 0.150 mol of an ideal gas is held constant at $77^0C$ while its volume is reduced to 25.0% of its initial volume. The initial pressure of the gas is 1.25 atm.
(a) Determine the work done by the gas.
(b) What is the change in its internal energy?
(c) Does the gas exchange heat with its surroundings?
If so, how much? Does the gas absorb or liberate heat?
Answer:
Calculate W and ΔU and then use the first law to calculate Q.
(a) W = $\int_{V_1}^{V_2}pdV$
pV = nRT → $p = \frac{nRT}{V}$
W = $\int_{V_1}^{V_2}\left(\frac{nRT}{V}\right)dV$
W = nRT$\int_{V_1}^{V_2}\left(\frac{nRT}{V}\right)dV$
W = nRT ln$\frac{V_1}{V_2}$
(work done during an isothermal process).
W = (0.150 mol)(8.3145 J/mol.K)(350 K) ln $\left(\frac{0.25V_1}{V_1}\right)=-605$ J
W for the gas is negative, since the volume decreases.
(b) ΔU= n$C_v\Delta T$ for any ideal gas process. ΔT = 0 (isothermal) so ΔU = 0
ΔU = 0 for any ideal gas process in which T doesn’t change.
(c) ΔU = Q − W, ΔU = 0 so Q = W= − 605 J .
(Q is negative; the gas liberates 605 J of heat to the surroundings.)
Q = n$C_v\Delta T$ is only for a constant volume process so doesn’t apply here. Q = n$C_p\Delta T$ is only for a constant pressure process so doesn’t apply here.
Q#19.28
An experimenter adds 970 J of heat to 1.75 mol of an ideal gas to heat it from 10.0 $^0C$ to 25.0 $^0C$ at constant pressure. The gas does +223 J of work during the expansion.
(a) Calculate the change in internal energy of the gas.
(b) Calculate $\gamma$ for the gas.
Answer:
$\Delta U = Q -W$. Apply Q = n$C_p\Delta T$ to calculate $C_p$.
Apply $\Delta U$ = n$C_v\Delta T$ to calculate $C_v$
$\gamma = \frac{C_p}{C_v}$
Given: $\Delta T=15.0^0C$ = 15.0 K. Since heat is added, Q = + 970 J
(a) $\Delta U = Q - W$ = +970 J - 223 J = 747 J
(b) $C_p=\frac{Q}{n\Delta T}$
$C_p=\frac{970 \ J}{(1.75 \ mol)(15.0 \ K)}$ = 37.0 J/mol.K
$C_v=\frac{\Delta U}{n\Delta T}$
$C_v=\frac{747 \ J}{(1.75 \ mol)(15.0 \ K)}$ = 28.5 J/mol.K and
$\gamma = \frac{C_p}{C_v}=\frac{37.0 \ J/mol.K}{28.5 \ J/mol.K}$ = 1.30
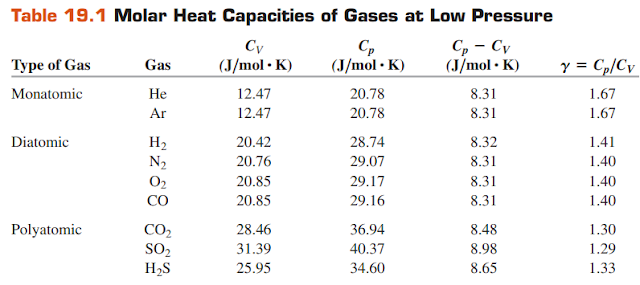
The value of γ we calculated is similar to the values given in Tables 19.1 for polyatomic gases.
Post a Comment for " Kinds of Thermodynamic Processes, Internal Energy of an Ideal Gas, and Heat Capacities of an Ideal Gas Problems and Solutions 4"