Q#19.37
One mole of ideal gas is slowly compressed to one-third of its original volume. In this compression, the work done on the gas has magnitude 600 J. For the gas, $C_v$ = 7R/2 .
(a) If the process is isothermal, what is the heat flow Q for the gas? Does heat flow into or out of the gas?
(b) If the process is isobaric, what is the change in internal energy of the gas? Does the internal energy increase or decrease?
Answer:
The compression does work on the gas, but the heat transferred and the internal energy change depend on the process by which the compression occurs. The ideal gas law and the first law of thermodynamics apply to the gas.
Q = ΔU + W, pV = nRT, and $C_v=C_p-R$
(a) This is an isothermal process for an ideal gas, so ΔU = 0 and Q = W Since the volume decreases (compression), W is negative and Q = −600 J. Since Q is negative, heat flows out of the gas.
(b) W = pΔV = nRΔT
$\Delta T = \frac{W}{nR}=\frac{-600 \ J}{(1 \ mola)(8.3145 \ J/mol.K)}=-72.2 \ K$
$C_v=C_p-R =\frac{5R}{2}=20.78 \ J/mol.K$
$\Delta U = nC_v\Delta T=(1 \ mol)(20.78 \ J/mol.K)(-72.2 \ K)=-1500 \ J$
Since ΔU is negative, the internal energy decreases.
In part (a) work is done on the gas, so heat must flow out of it for its temperature to remain the same. In (b) gas is compressed, so the molecules must slow down if the pressure is to remain the same, which means that the internal energy (and the temperature) must decrease.
Q#19.38
 |
| Fig. P19.38 |
CALC Figure P19.38 shows the pV-diagram for an isothermal expansion of 1.50 mol of an ideal gas, at a temperature of 15$^0C$
(a) What is the change in internal energy of the gas? Explain.
(b) Calculate the work done by (or on) the gas and the heat absorbed (or released) by the gas during the expansion.
Answer:
Apply ΔU = Q − W. For any process of an ideal gas, ΔU = n$C_v\Delta T$.
For an isothermal expansion,
W = nRT ln$\left(\frac{V_2}{V_1}\right)$ = nRT ln$\left(\frac{p_1}{p_2}\right)$
Given: T = (273.15 + 15) K = 288.15 K; $\frac{V_2}{V_1}=\frac{p_1}{p_2}=2.00$
(a) ΔU = 0 since ΔT = 0.
W = (1.50 mol)(8.3145 J/mol.K)(288.15 K) ln (2) = 2.49 $\times 10^3$ J
W > 0 and work is done by the gas. Since ΔU = 0, Q = W = +2.49 $\times 10^3$ J. Q > 0 so heat flows into the gas.
When the volume increases, W is positive.
Q#19.39
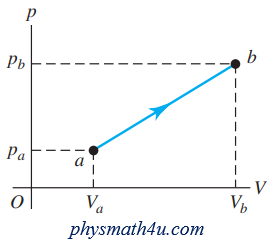
A quantity of air is taken from state a to state b along a path that is a straight line in the pV-diagram (Fig. P19.39).
 |
| Fig. P19.39 |
(a) In this process, does the temperature of the gas increase, decrease, or stay the same? Explain.
(b) If $V_a$ = 0.0700 $m^3$, $V_b$ = 0.1100 $m^3$, $p_a$ = 1.00 $\times 10^5$ Pa and $p_b$ = 1.40 $\times 10^5$ Pa, what is the work W done by the gas in this process? Assume that the gas may be treated as ideal.
Answer:
For an ideal gas, pV = nRT. The work done is the area under the path in the pV-diagram.
(a) The product pV increases and this indicates a temperature increase.
(b) The work is the area in the pV plane bounded by the blue line representing the process and the verticals at $V_a$ and $V_b$.
The area of this trapezoid is
$\frac{1}{2}(p_a+p_b)(V_b-V_a)=\frac{1}{2}(2.40 \times 10^5 \ Pa)(0.0400 \ m^3)=4800 \ J$
The work done is the average pressure, $\frac{1}{2}(p_a+p_b)$ times the volume increase.
Post a Comment for "The First Law of Thermodynamics Problems and Solutions"